Author: Cheryl Stratos
-
Helpful Hints:
Research what hospitals are the leaders in your type of cancer, review your options, and their statistics. US News and World Reports assesses hospitals every year at http://health.usnews.com/best-hospitals Clinical trials may produce better results than the standard of care. Visit http://www.clinicaltrials.gov/ Interview your oncologist to make sure you are comfortable with them because you’ll be…
-
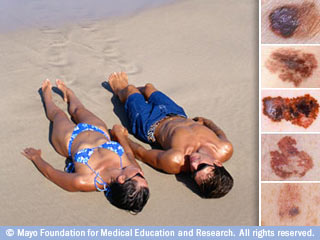
ABCDE Checklist
Follow this checklist for finding out whether a mole is more likely to be normal or a melanoma: Asymmetrical – the mole is not symmetrical, one half is different in shape from the other. Border – the border is ragged or notched. Most normal moles have regular borders Colors – while most normal moles have…
-
What are the symptoms of Malignant melanoma?
Malignant melanoma exists in a deeper layer of skin compared to other skins cancers. Experts say this is one of the reasons it is the most serious type of skin cancer. The deeper it starts from, the higher are the chances of it spreading. Patients mainly have melanomas on the back of the legs, arms…
-
What Is Skin Cancer? What Is Melanoma?
Melanoma is a malignant tumor of melanocytes. The tumors are generally found in the skin, but may also appear in the bowel and the eye (uveal melanoma). Melanoma is a type of skin cancer – one of the rarer types – but the cause of most skin cancer related deaths. Malignant melanoma is caused by…
-
Best Cancer Hospitals – US News & World Reports
Compare the best cancer hospitals. U.S. News evaluated nearly 886 hospitals for cancer treatment and ranked the top 50 that treat cancers such as leukemia, lymphoma, melanoma, breast, kidney, colon, prostate, pancreatic, head and neck, orthopedic, uterine and ovarian cancers. See also a list of oncologists and hospitals that are high performing in colon cancer surgery, https://health.usnews.com/best-hospitals/hospital-ratings/lung-cancer-surgery”,”_type”:”d82f1fd9-c1d7-315a-9519-34a46b1f526f”},”_type”:”809caec9-30e2-3666-8b71-b32ddbffc288″}”>lung cancer surgery, https://health.usnews.com/best-hospitals/hospital-ratings/prostate-cancer-surgery”,”_type”:”d82f1fd9-c1d7-315a-9519-34a46b1f526f”},”_type”:”809caec9-30e2-3666-8b71-b32ddbffc288″}”>prostate…
-
New Melanoma Treatment Might Delay Cancer Progression
SATURDAY, Sept. 29 (HealthDay News) — Researchers say they’ve discovered a two-drug combination that delays treatment resistance in patients with advanced melanoma. By targeting different points in the same growth-factor pathway, the kinase inhibitor drugs dabrafenib and trametinib postponed the development of drug resistance in patients with BRAF-positive metastatic melanoma, the study authors said. Melanoma…
-
Glaxo Two-Drug Melanoma Combo Slows Cancer in Study
GlaxoSmithKline Plc (GSK)’s combination of two experimental melanoma medicines slowed the cancer’s progress longer than a single-drug treatment, a study found. Patients taking Glaxo’s dabrafenib and trametinib together delayed tumors from progressing for 9.4 months, compared with 5.8 months for patients taking dabrafenib alone, according to the study of 162 patients. The trial was part…
-

-
Methods to Improve Adoptive T-Cell Therapy for Melanoma: IFN-γ Enhances Anticancer Responses of Cell Products for Infusion
Further development of adoptive T-cell therapy (ACT) with autologous tumor-infiltrating lymphocytes (TILs) has the potential to markedly change the long-term prognosis of patients with metastatic melanoma, and modifications of the original protocol that can improve its clinical efficacy are highly desirable. In this study, we demonstrated that a high in vitro tumor reactivity of infusion…
-
Engineering the Immune System to Fight Melanoma
ScienceDaily (Oct. 1, 2012) — Loyola University Medical Center has launched the first clinical trial in the Midwest of an experimental melanoma treatment that genetically engineers a patient’s immune system to fight the deadly cancer. A batch of the immune system’s killer T cells will be removed from the patient and genetically modified in a…
